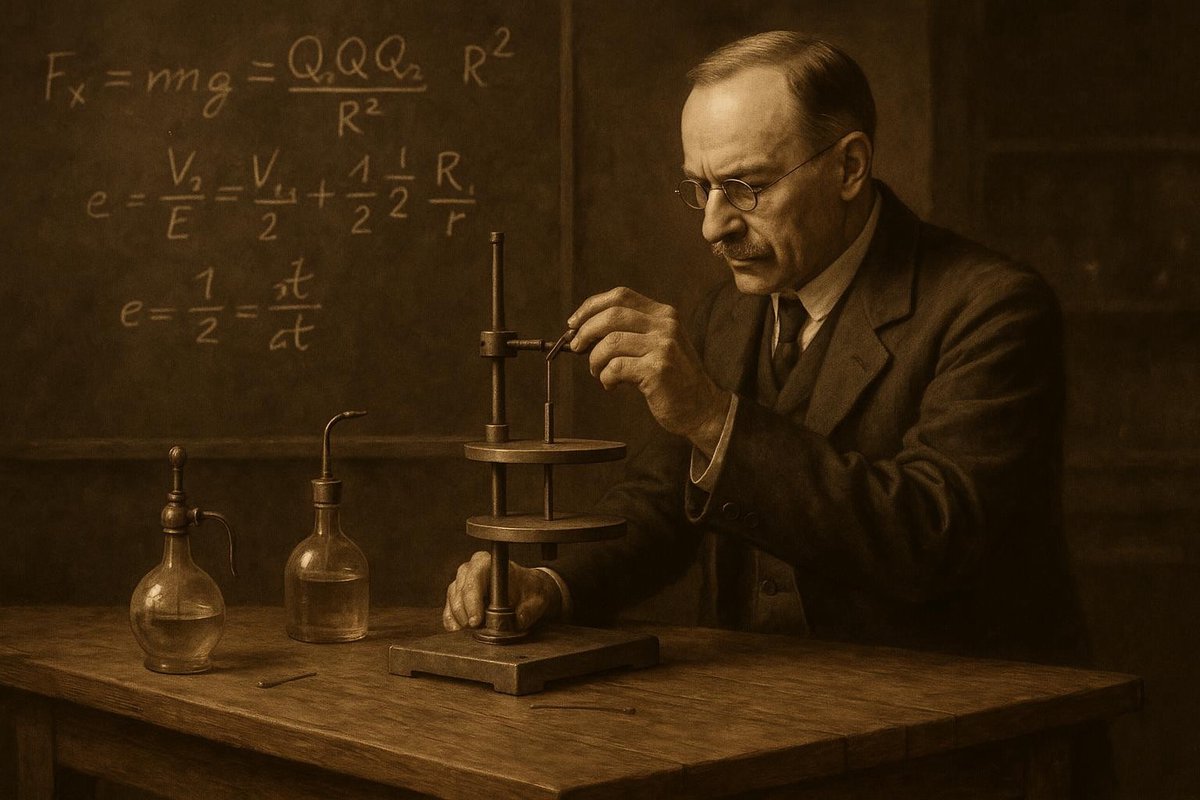
In the early 20th century, scientific curiosity was soaring to new heights. Among the many questions that perplexed physicists was the true nature of the electron’s charge. Enter Robert Millikan, whose meticulous Oil Drop Experiment would eventually change the course of physics forever. Interestingly, this experiment not only provided a definitive value for the electron’s charge but also challenged longstanding theories, paving the way for modern physics. How did Millikan achieve this monumental feat? Let’s dive into the details and unravel this scientific detective story.
The Ingenious Setup: How It Worked
Millikan’s Oil Drop Experiment was a marvel of scientific apparatus and methodical precision. At its core, the experiment involved balancing the gravitational and electric forces on tiny oil droplets. But how did Millikan do it?
- He used a chamber with two parallel metal plates to create an electric field.
- Oil droplets were sprayed into the chamber, where their movement could be controlled by adjusting the electric field.
- By carefully measuring the rate at which the droplets rose or fell, Millikan could calculate the charge on each droplet.
The elegance of this setup lay in its simplicity. Much like a detective piecing together clues, Millikan meticulously adjusted variables and recorded data to deduce the charge. Imagine balancing a pencil on your fingertip—it’s a delicate act requiring precision and patience. Millikan’s ingenuity lay in transforming this balance into a quantitative measurement.
What Millikan Set Out to Prove
Millikan’s primary goal was to determine the exact charge of an electron. At the time, the electron was a mysterious entity, with its charge seen as elusive and enigmatic.
- He aimed to provide conclusive evidence of the quantization of electric charge.
- Millikan wanted to test the hypothesis that charge occurs in discrete units—multiples of a fundamental charge.
- He hoped to resolve debates about the nature of atomic physics.
To think of it in simpler terms, Millikan was attempting to weigh the invisible. Consider trying to measure the weight of a single grain of sand while standing on a windy beach—a daunting task for anyone. Yet, through this experiment, Millikan aimed to shine a light on the most fundamental building blocks of matter, setting the stage for future breakthroughs in atomic theory.
Unexpected Results: Challenging Prior Theories
As time went on, Millikan’s findings began to stir the scientific community. His results were surprisingly precise, revealing the charge of an electron to five significant figures.
- The measured charge was consistently a multiple of approximately 1.6 x 10^-19 coulombs.
- This directly supported the theory of quantized charge, which was revolutionary at the time.
- Millikan’s data contradicted earlier beliefs that electric charge could exist in any arbitrary amount.
It was like discovering a hidden pattern in what was thought to be chaos. Imagine expecting to find scattered pebbles on a beach but instead uncovering a perfectly symmetrical mosaic. No wonder Millikan’s work was seen as groundbreaking. His meticulous attention to detail offered clarity where there had been confusion, much like the work of an artist revealing a masterpiece behind what seemed like random strokes.
Lasting Impact: Millikan’s Legacy
Of course, the implications of Millikan’s success were vast and far-reaching. His experiment became a cornerstone in the field of physics.
- It provided experimental support for the existence of quantized charge, influencing quantum mechanics.
- The methodology set new standards for precision in experimental physics.
- Millikan’s work paved the way for further exploration into atomic and subatomic particles.
Imagine erecting a lighthouse in a stormy sea—guiding countless ships safely home. Millikan’s experiment similarly guided physicists toward a deeper understanding of the atomic world. His legacy endures as a testament to the power of curiosity and dedication in unraveling the mysteries of nature.
As we reflect on the past, Millikan’s Oil Drop Experiment remains a shining example of how science evolves through challenging assumptions and rigorous inquiry. The clarity it brought to the atomic world revolutionized our understanding, shaping the course of modern physics.
Fuel Someone Else’s Curiosity
If this exploration into the fascinating world of Millikan’s Oil Drop Experiment sparked your curiosity, why not share it with others? Science grows richer and more vibrant with every conversation. Share this article and let others embark on this journey of discovery alongside you.

Leave a Reply